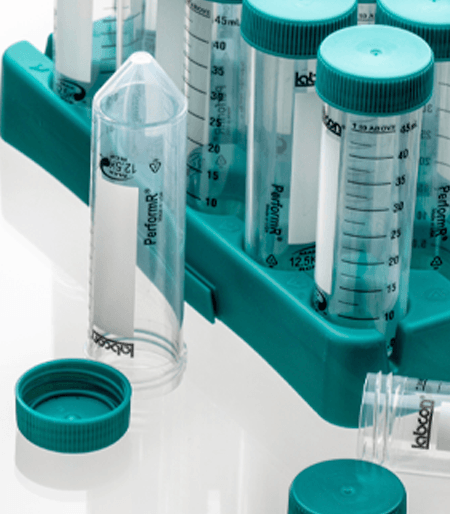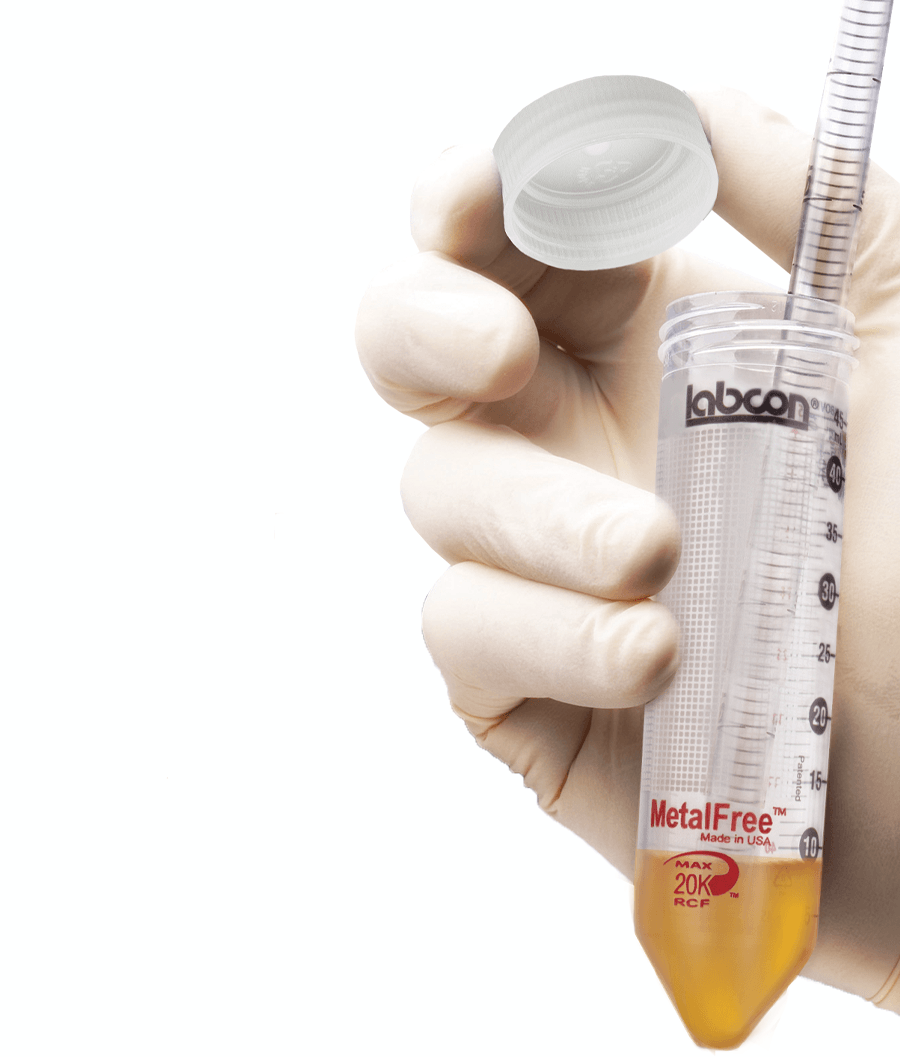
MetalFree® Centrifuge Tubes
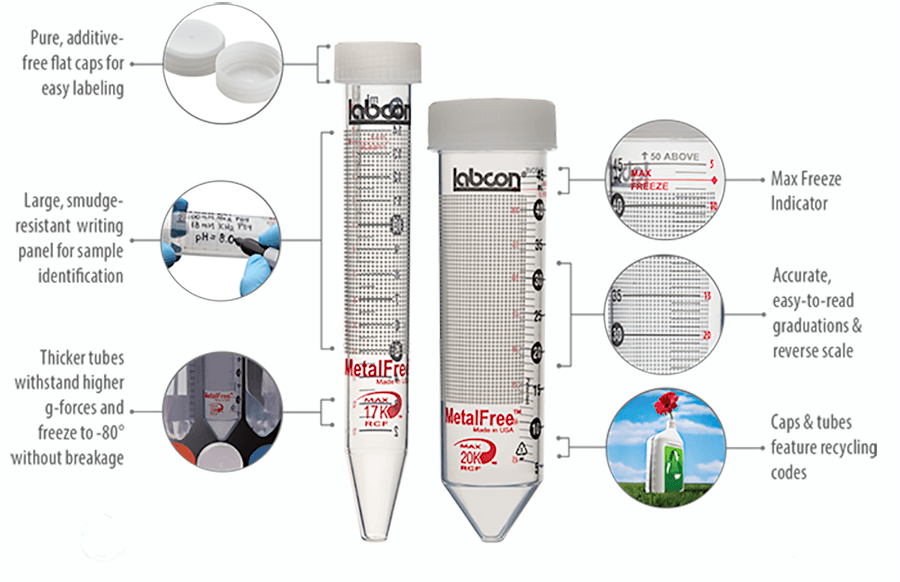
Our MetalFree® tubes are produced to ensure that key trace metals are kept below one part per billion (1ppb). They can be used for storage and analysis tasks that require extremely low levels of most common metals. These tubes are produced with a proprietary process that virtually eliminates common metals.
Far cleaner than metal free tubes offering a simple 1 part per million (1ppm) certification these tubes are unique. Developed in our own lab and using an Inductively Coupled Plasma Mass Spectroscopy (ICP) system these tubes are free of more than 40 common trace metals to a detection level of 1 part per billion (10-9).
Unmatched Purity
Our MetalFree® tubes are not only free of ultra low levels of trace metals. These tubes are also lot tested and certified free of detectable Nuclease, Endotoxin, and ATP contamination.
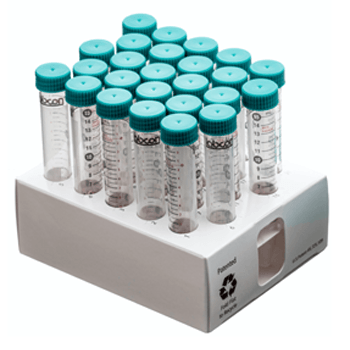
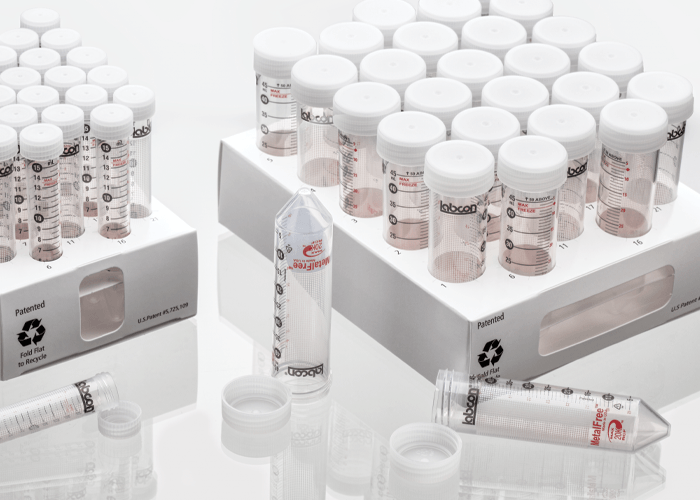
MetalFree® tubes are available in 15mL, 50mL and sizes in our Patented fiber racks or in Loose bags. Our MetalFree® tubes are also available in a snap cap 1.5mL microcentrifuge tube. All our sterile products use medical grade packaging and are radiation sterilized following a validated ISO 11137 method to a sterility assurance level of 10-6.
Tested for 43 Common Metals
MetalFree® centrifuge tubes are cleaner than lower quality brands of tubes. Our MetalFree® tubes are independently lab tested using ICP-MS to ensure they are free of 43 metals to a detection level of 1 part per billion.











































Our MetalFree® tubes are made with ultra clean resins for the tube and cap and processed to ensure that common trace metals are kept below an ICP-MS detection level of one part per billion (10-9). These tubes are perfect for storage of samples and for applications requiring the assurance of low background metals.
Metals Tested
All package versions are sterilized with a validated and approved ISO 11137 medical grade method to an SAL of 10-6. Racked tubes are packaged in recyclable fiber racks.
Autoclavable (122°C) with caps attached. Freezable (-80°C).
| Endotoxin Free (Non pyrogenic) Product samples are exposed to endotoxin-free water and the resulting extraction fluid is tested for contamination using the kinetic turbidimetric Limulus Amoebocyte Lysate (LAL) assay protocol and USP guidelines. All products tested must display less than 0.05 EU/ml to be certified free of endotoxin. |
|
| Nuclease Free (RNase/DNase) Product samples are exposed to nuclease-free water and the resulting extraction fluid is tested for nuclease activity on commercially available 7.5 kb Poly(A) tailed RNA (1µg) and HindIII-digested DNA (1µg) with a one hour 37°C incubation in appropriate buffers. Results are visualized on an agarose gel with appropriate positive and negative controls. Extraction fluid samples must show no degradation of the nucleic acids by the extraction fluid has occurred for the product to be certified as RNase-free and DNase-free |
|
| Adenosine Triphosphate (ATP) Product sample surfaces are tested for the presence of adenosine triphosphate (ATP) using a controlled bioluminescence reaction to detect contamination. Luminescence data is compared to results generated by ATP-free surfaces and surfaces with known amounts of ATP as a positive control. The relative light units result must indicate less than 2 X 10-12 mg/µl of ATP for the product to be certified as ATP free. |
|
| Heavy Metals Free Heavy metals have been tested for using the prescribed USP method and confirmed to have levels lower than 1 part per million (1ppm) |
|
| Metal Free Our ultra-low metal products are tested with an ICP-MS method to confirm that the following elements fall below 1 part per billion (1ppb): Calcium, Magnesium, Zinc, Iron, Manganese, Copper, Aluminum, Silicon, Nickel, Vanadium, Sodium, Phosphorous, Cobalt, Chromium, Potassium, Lithium, Lead, Selenium, Cadmium, Mercury, Arsenic, Beryllium, Silver, Gold, Barium, Bismuth, Cerium, Caesium, Gallium, Germanium, Molybdenum, Niobium, Rubidium, Rhenium, Antimony, Tin, Strontium, Tantalum, Titanium, Thallium, Tungsten, Yttrium, Zirconium. |
|
| Validated Sterilization Labcon products are sterilized by radiation sterilization within a dose range of 12-35 kGy. The dose range necessary for the stated sterility assurance level is continuously audited through quarterly bio-burden and sterility validation studies performed according to the ANSI/AAMI/ISO 11137 standard by an independent laboratory. This dosage is sufficient to guarantee a sterility assurance level of 10-6. |
| Bovine Spongiform Encephalopathy-Transmissible Spongiform Encephalopathy These products contain resins that are processed under conditions proven to exceed the European Union standard as listed in the 22nd Commission Directive EC 98/16/EC of March 5th, 1998 as annexed to council Directive EC 76/768/EEC and further Amendment 419 Annex II of 12 June 2001. |
|
| Medical Grade (USP) U.S Pharmacoepia Methods and Guidelines (U.S.P Class VI) are used if applicable. We only use medical grade resins and pre-test all resins for contaminants prior to use. Resins are compliant with FDA CFR title 21-177.1520, 178.3295, 178.3297. |
|
| California Prop 65 No Labcon manufactured disposables contain any of the “listed chemicals” as referenced in the California Safe Drinking Water and Toxic Enforcement Act of 1986, (Prop 65) as revised May 25, 2018. |
|
| Phthalates & Oleamide All our resins are medical grade and are certified free of Bisphenol A (BPA), Oleamide, DiHEMDA, and Phthalates. |
|
| Substances of Very High Concern & REACH All Labcon products are compliant with RoHS Directive 2002/95/EC/-2011/65-2015/863, are free of Substances of Very High Concern (SVHC), and are EU REACH Regulation (EC) No 1907/2006 compliant. |
|
| Origin These products are Made in USA and all components meet the requirements for US origin under the NAFTA agreement. |
| U.S. FDA Registered Labcon is a U.S. FDA registered medical device manufacturer. Our facility is registered by the U.S. government to comply with CFR21 GMP regulations for manufacturing medical devices. |
|
| CE Compliant As applicable Labcon products meet the requirements for CE marking under regulation 2017/746. |
|
| ISO 9001 Quality Registration Labcon has been registered to the ISO 9001 quality management system standar since 1997 and maintains registration to ISO 9001:2015. |
| Material | Proprietary Virgin Polypropylene |
| Grade | Medical |
| Rating | USP Class VI |
| Colors | Clear |
| Length | Various |
| Fits | Most popular centrifuges |
| Approved Use | Medical, Research, Industrial, Food & Beverage |
| Lot Expiration | 6 years |
| Sterile Expiration | 5 years |
Fiber Rack
These patented collapsable recyclable fiber racks hold 25 tubes and can be recycled like standard cardboard. The clean SBS material is printed with numbers at each well for ease of use. A view port in the ends allows you to see the fluid volume when filling one tube. Available sterilized by irradiation.