H-Pette™ Tool for Blood Smears
The H-Pette™ is a safe, time-saving, all-in-one tool for creating blood smears for differential slides
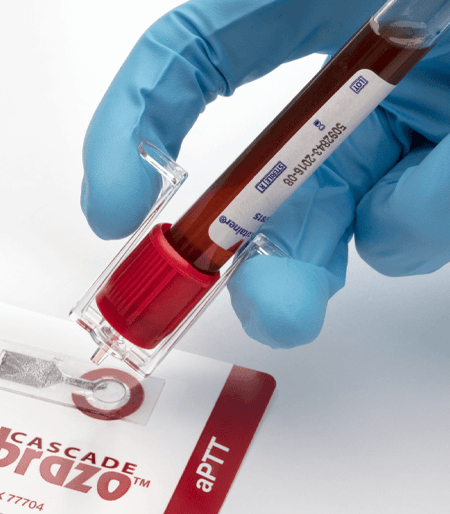
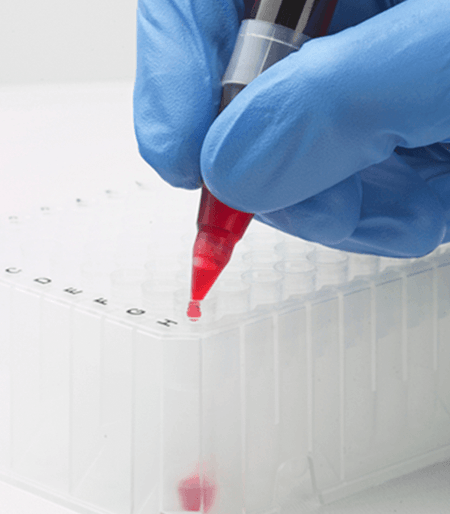
Labcon’s H-Pette™ Dispenser makes blood slide preparation safe and easy. Simply insert the H-Pette™ directly into the stopper of any size blood collection tube, dispense one drop onto a glass slide, and use the integral blade to smear the drop across the slide.


Easy Differential Smears
H-Pette™ streamlines the process of making slide smears. Simply push to release one drop and and then slide the H-Pette™ blade across the drop for a perfect smear. No need to use another glass slide. And H-Pette™ stays attached to the tube should you need subsequent smears.
H-Pette™ is a one piece molded tool with an integral spreader blade. It inserts onto all popular blood collection tubes. Since you don’t have to open the primary sample tube, you greatly reduce the risk of lab-acquired infection by minimizing aerosols and exposure to blood borne pathogens.
H-Pette™ simplifies blood smear preparation by cutting out several steps, thereby improving efficiency, saving time, and reducing wasted materials and blood samples. Using the H-Pette™ eliminates the need for sticks, capillaries, or syringes to dispense blood on the slide. And H-Pette™ requires no special training, no special fittings or inserts.
Available in a resealable package of 100 or 5 packs of 100.
| Endotoxin Free (Non pyrogenic) Product samples are exposed to endotoxin-free water and the resulting extraction fluid is tested for contamination using the kinetic turbidimetric Limulus Amoebocyte Lysate (LAL) assay protocol and USP guidelines. All products tested must display less than 0.05 EU/ml to be certified free of endotoxin. |
|
| Nuclease Free (RNase/DNase) Product samples are exposed to nuclease-free water and the resulting extraction fluid is tested for nuclease activity on commercially available 7.5 kb Poly(A) tailed RNA (1µg) and HindIII-digested DNA (1µg) with a one hour 37°C incubation in appropriate buffers. Results are visualized on an agarose gel with appropriate positive and negative controls. Extraction fluid samples must show no degradation of the nucleic acids by the extraction fluid has occurred for the product to be certified as RNase-free and DNase-free |
|
| Adenosine Triphosphate (ATP) Product sample surfaces are tested for the presence of adenosine triphosphate (ATP) using a controlled bioluminescence reaction to detect contamination. Luminescence data is compared to results generated by ATP-free surfaces and surfaces with known amounts of ATP as a positive control. The relative light units result must indicate less than 2 X 10-12 mg/µl of ATP for the product to be certified as ATP free. |
| Bovine Spongiform Encephalopathy-Transmissible Spongiform Encephalopathy These products contain resins that are processed under conditions proven to exceed the European Union standard as listed in the 22nd Commission Directive EC 98/16/EC of March 5th, 1998 as annexed to council Directive EC 76/768/EEC and further Amendment 419 Annex II of 12 June 2001. |
|
| Medical Grade (USP) U.S Pharmacoepia Methods and Guidelines (U.S.P Class VI) are used if applicable. We only use medical grade resins and pre-test all resins for contaminants prior to use. Resins are compliant with FDA CFR title 21-177.1520, 178.3295, 178.3297. |
|
| California Prop 65 No Labcon manufactured disposables contain any of the “listed chemicals” as referenced in the California Safe Drinking Water and Toxic Enforcement Act of 1986, (Prop 65) as revised May 25, 2018. |
|
| Phthalates & Oleamide All our resins are medical grade and are certified free of Bisphenol A (BPA), Oleamide, DiHEMDA, and Phthalates. |
|
| Substances of Very High Concern & REACH All Labcon products are compliant with RoHS Directive 2002/95/EC/-2011/65-2015/863, are free of Substances of Very High Concern (SVHC), and are EU REACH Regulation (EC) No 1907/2006 compliant. |
|
| Origin These products are Made in USA and all components meet the requirements for US origin under the NAFTA agreement. |
| U.S. FDA Registered Labcon is a U.S. FDA registered medical device manufacturer. Our facility is registered by the U.S. government to comply with CFR21 GMP regulations for manufacturing medical devices. |
|
| CE Compliant As applicable Labcon products meet the requirements for CE marking. |
|
| ISO 9001 Quality Registration Labcon has been registered to the ISO 9001 quality management system standar since 1997 and maintains registration to ISO 9001:2015. |
| Material | 100% Virgin Polystyrene |
| Grade | Medical |
| Rating | USP Class VI |
| Colors | Natural (Clear) |
| Fits | Most popular blood collection tubes |
| Approved Use | Medical, Clinical |
| Lot Expiration | 6 years |